What We Do
Together, We Heal
What We Do
Our Vision, Mission & Values
Our Vision
To make PACER the voice of patients in clinical research.
Our Mission
To create expert patients who can effectively represent the patient voice in the clinical research process.
Our Core Values
• Patient Centricity
• Collaboration
• Communication
• Engagement
• Empowerment
• Integrity
What We Do

Education & Training
Workshops for patients, caregivers, survivors & PAG leaders

Building Patient Advocates
Capacity-building for PAGs to influence policy and research

Creative Awareness
Nukkad natak, survivor art, school & community engagement

Policy & Advocacy
Consultations with regulators, ethics committees & research institutions, with PAGs as equal stakeholders

Publications & Resources
Toolkits, patient guides, advocacy materials for PAGs & families

Education & Training
Workshops for patients, caregivers, survivors & PAG leaders

Building Patient Advocates
Capacity-building for PAGs to influence policy and research

Creative Awareness
Nukkad natak, survivor art, school & community engagement

Policy & Advocacy
Consultations with regulators, ethics committees & research institutions, with PAGs as equal stakeholders

Publications & Resources
Toolkits, patient guides, advocacy materials for PAGs & families
Who We Are

CanKids…KidsCan
India's only national NGO working across the entire spectrum of childhood cancer care, providing comprehensive support from diagnosis to survivorship.
National Coverage
APAR Health
A health think-tank and implementation partner working to improve research, policy, and advocacy in India through evidence-based solutions.
Policy & Research
Patient Advocates & PAGs
Survivors, caregivers, and advocacy groups who co-create PACER's agenda and drive its implementation with lived experience and passion.
Community Driven
Clinical Research Workshops
A comprehensive series of workshops focused on clinical research awareness, ethics, and patient advocacy in healthcare
Workshop Series Overview
Our Impact
500
10,000
50
25
Our Impact
500
10,000
50
25
From Patient Navigator to Patient Advocate – Improving Healthcare outcomes through evidence based advocacy research
- 25th November 2023
- The Leela Palace, Chennai
Key Components Discussed
- Engagement of PAGs in drug development phases - How PAGs can help patients participate Research and hold hand for patients rights in research.




Good Clinical Practice & Research Ethics
- 15th June 2023
- Amrita Institute of Medical Sciences, Faridabad
Key Components Discussed
- How Patient Advocate Groups (PAGs) can Uphold the ethical principles and avoid conflicts of interest and bias.




Awareness on Clinical Research
- 31st March 2023
- Medanta, The Medicity, Gurugram
Key Components Discussed
- What is clinical research - Informed consents - Ethical, legal & regulatory framework - Importance of Clinical research for patient advocates










Workshop Series Overview
This comprehensive workshop series provided essential knowledge and skills for patient advocates, covering clinical research fundamentals, ethical practices, regulatory frameworks, and patient-centric approaches to improve healthcare outcomes.
Workshop Statistics
Workshop 1: Impact Analysis of Clinical Research Awareness
Awareness on Clinical Research
- 31st March 2023
- Medanta, The Medicity, Gurugram
Key Components Discussed
- What is clinical research - Informed consents - Ethical, legal & regulatory framework - Importance of Clinical research for patient advocates










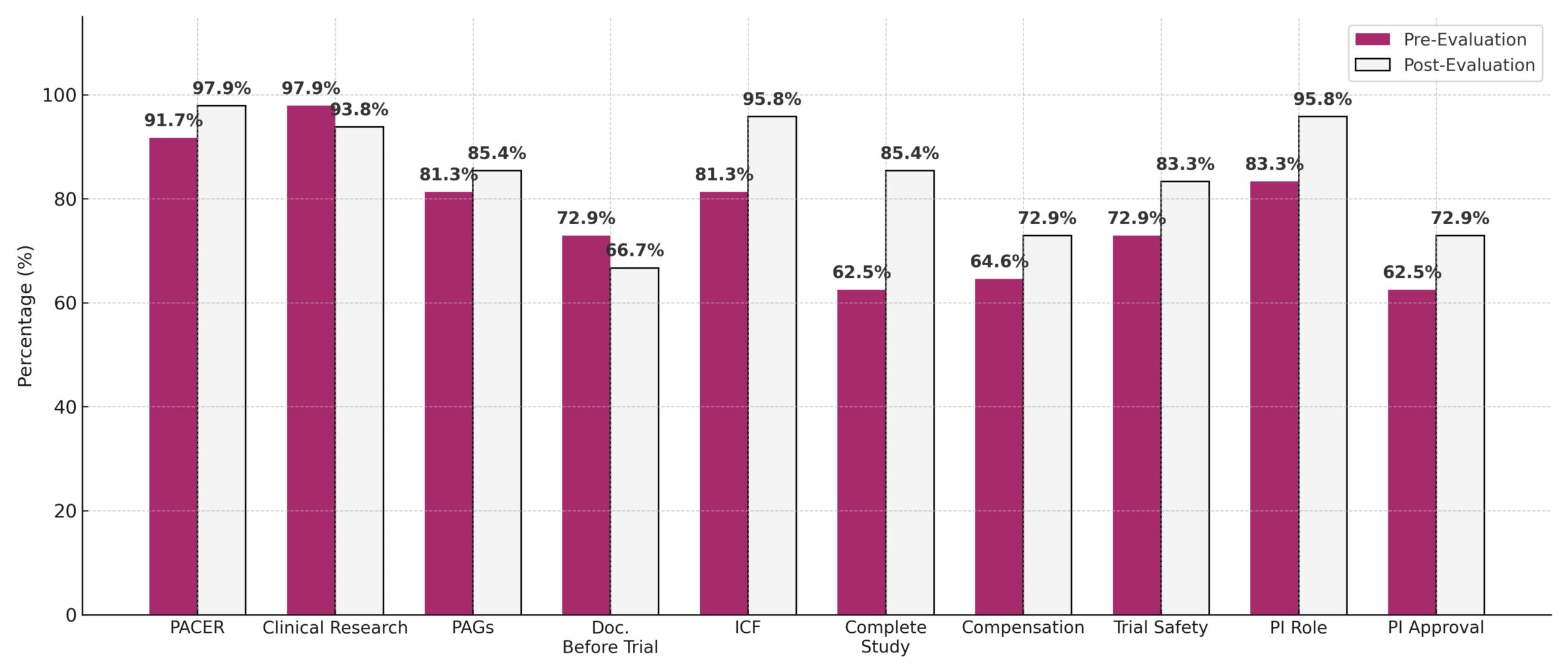
- PACER awareness increased: 91.7% → 97.9%
- ICF understanding improved significantly: 81.3% → 95.8% (p = 0.039) ★
- Study completion awareness rose: 62.5% → 85.4% (p = 0.013) ★
- PI role clarity improved: 83.3% → 95.8% (p = 0.031) ★
- Slight drop in clinical research awareness: 97.9% → 93.8%
🗒 Note:
★ = Statistically significant improvement (p < 0.05)
A total of 20 PAGs represented by 48 participants, active in areas of pediatric cancer, breast cancer, multiple myeloma, type I diabetes, spinal muscular atrophy, sickle cell disease, and inflammatory bowel diseases, participated. Among 48 participants 30 successfully completed the online course (multiple-choice question evaluation score cut-off >70%), attaining an average score of 23.9 ± 2.1 out of 30. Overall, 48 participants attended workshop 1 and 45 workshop 2, with 140 participants joining the focus group discussion (FGD). An overall improvement of 9.4% (2 = 46.173; p < 0.001) for workshop 1 and 8.2% (2 = 25.412; p < 0.001) for workshop 2 was seen in knowledge gain about clinical research. The FGD raised issues such as misleading information from research teams, unethical recruitment, incomprehensible information sheets, and limited trial-related knowledge fostering fear of participation in clinical research.










Awareness on Clinical Reaserach
- 31st March 2023
- Medanta, The Medicity, Gurugram
- Key Components Discussed: What is clinical research? Informed consents. Ethical, legal & regulatory framework. Importance of Clinical research for patient advocates.










Education
Awareness On Clinical Research
Learn essential skills for effective patient advocacy and healthcare navigation.
- March 15, 2025 | 10:00 AM – 4:00 PM
- PACER Headquarters, New Delhi (In-Person Event)
- 45/60 Registered | 15 spots left


