Events Around the Corner
- Ongoing
FERCI in collaboration with PACER
Online Patient-centric Workshop
on 31st January, 2026
- The workshop will highlight the experiences of non-scientist members of Ethics Committees and patient groups, and the training of non-scientists.
-
Registration fee: Rs. 300/-
For FERCI Members: Rs. 200/-

Health Magazine, Hamari Sehat
- Past Event
Clinical Trials as a Care Option
- Event completed successfully
- Thank you for joining us
- Stay tuned for more events
- Ongoing
CTRI orientation program
- Happening Now!
- Live Online
- Register Now to Participate
- Ongoing Study
PVPI ADR Reporting Training
- Event completed successfully
- Thank you for joining us
- Stay tuned for more events
- Past Event
5th July event
- Event completed successfully
- Thank you for joining us
- Stay tuned for more events
Milestones in Advocacy & Training






Workshop Statistics
Workshop 6: Clinical Trial as a clinical care option - 28th November 2025
Event: 9th PHOSSCON (parallel to PHOCON 2025)
Venue: Kasturba Medical College & Hospital, Manipal
Focus: To highlight clinical trials as a safe and ethical care option by helping patients understand access, consent, benefits, and responsibilities.








Workshop 5: Empowering Patients as Partners in Clinical Research






















Workshop 4: Orientation towards Patient-Centric Research
Patient-Centered Approach in Clinical Research
- 29th January 2024
- Medanta, The Medicity, Gurugram
Key Components Discussed
- Rights of Patients - Ethics Committee and Role of Lay person in review research proposal - Digital Personal Data Protection Act, 2023



The workshop on Orientation Towards Patient-Centric Research led to increased awareness of the importance of involving patients throughout clinical studies. Participants gained knowledge about ethical standards, patient rights, and communication strategies. Hands-on activities improved their ability to design patient-friendly protocols, and feedback reflected greater confidence in applying a patient-focused approach in future research. The session also emphasized transparency, informed consent, and post-trial patient engagement. Overall, it fostered a strong foundation for ethical and empathetic clinical research practices.
Workshop 3: Breaking Barriers to Participation
From Patient Navigator to Patient Advocate – Improving Healthcare outcomes through evidence based advocacy research
- 25th November 2023
- The Leela Palace, Chennai
Key Components Discussed
- Engagement of PAGs in drug development phases - How PAGs can help patients participate Research and hold hand for patients rights in research.




Among 185 participants, 97.8% were aware about clinical research/trials and 90.3% understood clinical trials are conducted before any drug reaches market. Participants identified safety concerns (82.2%), limited knowledge (67.6%), and fear (58.9%) as key reasons for barriers for participation. Participants prioritized increased patient participation (59.5%), availability of more clinical trials (54.6%), and substantial investment in healthcare (51.9%) as key drivers for developing India’s healthcare system. About 88.6% of participants believed that involvement of PAGs in clinical trials will benefit the patients. The FGD revealed a significant progress by PAGs. The active involvement of PAGs led to their inclusion on ethics committees and helped enroll 14 patients in clinical trials within just six months.
Workshop 2: Strengthening Research Ethics Awareness
Good Clinical Practice & Research Ethics
- 15th June 2023
- Amrita Institute of Medical Sciences, Faridabad
Key Components Discussed
- How Patient Advocate Groups (PAGs) can Uphold the ethical principles and avoid conflicts of interest and bias.




The workshop was attended by 116 participants. Of these 91 consented to participate in questionnaire evaluation that assessed participants’ knowledge on ethics committee (EC) functionality, research ethics and data confidentiality. Pre-workshop evaluations highlighted knowledge gaps. Only 16.5% were familiar with the primary ethical consideration for vulnerable populations and 69.2% were knowledgeable about data governance. Post-workshop evaluations demonstrated significant overall response improvement of 5.4% (2=13.890; p<0.001). The understanding of ethical considerations for vulnerable populations rose by 15.4% (p=0.007), and knowledge of data privacy regulations improved by 11.0% (p=0.041).
Workshop 1: Impact Analysis of Clinical Research Awareness
Awareness on Clinical Research
- 31st March 2023
- Medanta, The Medicity, Gurugram
Key Components Discussed
- What is clinical research - Informed consents - Ethical, legal & regulatory framework - Importance of Clinical research for patient advocates










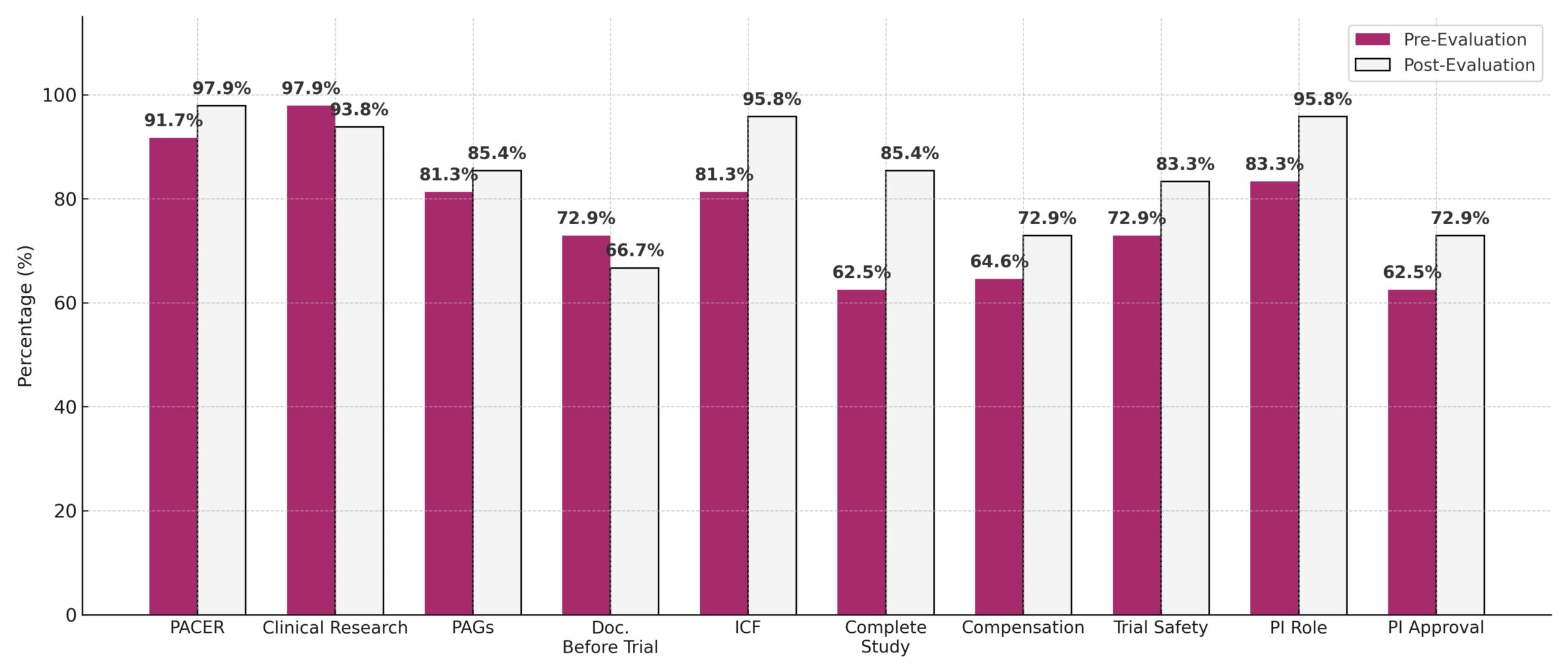
- PACER awareness increased: 91.7% → 97.9%
- ICF understanding improved significantly: 81.3% → 95.8% (p = 0.039) ★
- Study completion awareness rose: 62.5% → 85.4% (p = 0.013) ★
- PI role clarity improved: 83.3% → 95.8% (p = 0.031) ★
- Slight drop in clinical research awareness: 97.9% → 93.8%
🗒 Note:
★ = Statistically significant improvement (p < 0.05)
A total of 20 PAGs represented by 48 participants, active in areas of pediatric cancer, breast cancer, multiple myeloma, type I diabetes, spinal muscular atrophy, sickle cell disease, and inflammatory bowel diseases, participated. Among 48 participants 30 successfully completed the online course (multiple-choice question evaluation score cut-off >70%), attaining an average score of 23.9 ± 2.1 out of 30. Overall, 48 participants attended workshop 1 and 45 workshop 2, with 140 participants joining the focus group discussion (FGD). An overall improvement of 9.4% (2 = 46.173; p < 0.001) for workshop 1 and 8.2% (2 = 25.412; p < 0.001) for workshop 2 was seen in knowledge gain about clinical research. The FGD raised issues such as misleading information from research teams, unethical recruitment, incomprehensible information sheets, and limited trial-related knowledge fostering fear of participation in clinical research.


